Explain Three Differences Between Exothermic and Endothermic Reactions
Combustion and oxidation are the more common examples of this. In both reaction there is significant changes in temperatures.

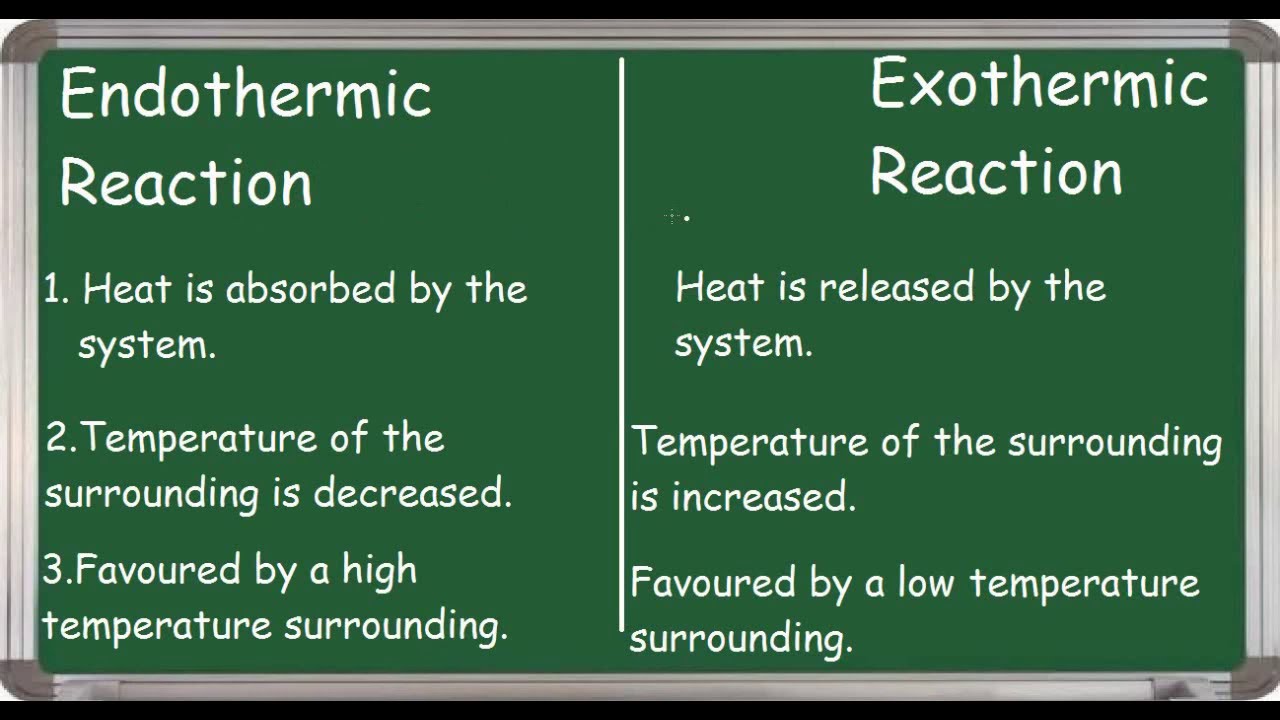
Exothermic Reactions Release Energy Endothermic Reactions Consume Energy Exothermic Reaction Homeschool Science Chemistry
Chemical reactions can be classified into two based on the absorption or release of heat energy.

. When energy is absorbed in an endothermic reaction the temperature decreases. Therefore the change in enthalpy is negative and heat is released to the surroundings. The endothermic reactions are when the system takes up the energy in the form of light or heat.
When energy is released in an exothermic reaction the temperature of the reaction mixture increases. ΔH rxn is positive. Chemical reactions can be divided into two groups as endothermic reactions and exothermic reactions according to the energy transfer between the surrounding and the system where the reaction is taking place.
In exothermic reactions the enthalpy change is always negative while in endothermic reactions the enthalpy change is always positive. Exothermic Reactions Endothermic Reactions. Work must be done in order to get these reactions to occur.
On the other hand an exothermic reaction releases energy into the surrounding of the system. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Similarities between Exothermic and Endothermic Reactions In both reactions a new substance is formed.
One of your salts generated an endothermic reaction with water while the other salt generated an exothermic reaction with water. These are endothermic reactions. Heat is released by the system into the surroundings.
The end products are stable in exothermic reactions. Some other differences between these types of chemical reactions are tabulated below. Salt A is ammonium nitrate and Salt B is calcium chloride.
Endothermic reactions are those that absorb heat from. A popular example of endothermic chemical reaction is photosynthesis. Heat is released by the reaction to surroundings.
The difference between. If K c increases with an increase in temperature the reaction to shifts to the right. In the lab exothermic reactions produce heat or may even be explosive.
Photosynthesis evaporation sublimation and melting ice are great examples. During this process plants absorb energy from the. This is due to the releasing and absorption of heat energy in the reactions respectively.
Exothermic reactions are chemical changes that release heat. Explore the differences. When investigating exothermic and.
Endothermic and Exothermic Reactions. There are other chemical reactions that must absorb energy in order to proceed. Endothermic reactions absorb heat to bring on a chemical change.
In both reactions energy is involved. Thus in order to react they absorb the energy from the environment. If the temperature of the mixture has gone up the reaction is exothermic.
Heat is absorbed from the surroundings. Let me first reveal the identity of your salts. Conclusion this esothermic and endothermic reactions can be classified on the basis of the transfer of energy between the system and the surrounding environment.
During this process plants observes energy from the Sun and convert it into carbon. Thats because you were given two different salts. Endothermic reactions cannot occur spontaneously.
A popular example of an endothermic chemical reaction is photosynthesis. In any physical or chemical process energy is neither created nor destroyed. In endothermic processes reactants possess lower potential energy than the product.
While the exothermic reaction releases energy into the surrounding from the system. In endothermic reactions the system gains heat as the surroundings cool down and in exothermic reactions the system loses heat as the surroundings heat up. 5 rows Exothermic Reactions The opposite of an endothermic reaction is an exothermic.
In simple terms the endothermic reactions absorb energy from the surrounding that is in the form of heat. 5 rows This is on the basis of release or taking the energy in the form of sound light cold or heat. ΔH rxn is negative.
If the temperature of the mixture has gone down the reaction is endothermic. Exothermic reaction In an exothermic reaction the total energy of the products is less than the total energy of the reactants. In contrast exothermic systems give up heat or light energy as the reaction proceeds.
In order to categorize a particular chemical reaction as endothermic or exothermic we can. In the presence of water a strong acid will dissociate quickly and release heat so it is an exothermic reaction. Law of conservation of energy.
The main difference is that exothermal reactions release energy in the surroundings while an endothermic reaction absorbs energy from the surroundings. In simple terms the endothermic reactions absorbs energy from the surrounding that is in the form of heat. There are two methods for distinguishing between exothermic and endothermic reactions.
If K c increases with a decreases in temperature the reaction to shifts to the right. In both reactions a chemical change takes place. The quantity of heat required to raise the temperature of 1 gram of water by 1C.
This can be used to classify. If K c decreases with an increase in temperature the reaction shifts to the left. Heat is absorbed by the system from the surroundings.
Main Difference Endothermic vs Exothermic Reactions. As the names suggest the primary difference between endothermic and exothermic reactions is that the former absorbs heat from the surroundings whereas the latter involves a release of heat. Heat is absorbed by reactants to form products.
As a result the surroundings get cold.

7 Difference Between Exothermic And Endothermic Reaction With Examples Viva Differences

Difference Between Endothermic And Exothermic Reactions Chemistry
Difference Between Endothermic And Exothermic Reactions Definition Properties Examples
No comments for "Explain Three Differences Between Exothermic and Endothermic Reactions"
Post a Comment